In a long ago meme in blogland, people offered to write posts about things that they don’t usually talk about. One of my friends going through old LJ posts asked me to write about the Standard Model of Physics. So here we go…
“At this final stage you teach me that this wondrous and multicoloured universe can be reduced to the atom and that the atom itself can be reduced to the electron… So that science that was to teach me everything ends up in a hypothesis, that lucidity founders in metaphor, that uncertainty is resolved in a work of art.” Albert Camus, The Myth of Sisyphus, 1942
I didn’t know that I had a favourite sub-atomic particle until I was teaching a class on the Standard Model. Then I found myself at the front of the class, dressed in a suit, and hopping up and down. “Oh!” I clapped my hands, childlike! “This is the best part!” I suspect that there were a few raised eyebrows, but I was in Physics-Land, so I’m not entirely sure. I did catch myself, however, and sheepishly faced the classroom. “Gluons,” I said, “are really cool.”
The Gluon, you see, is the particle that holds everything together. I’d love to take you there, but we need to tunnel down through some levels of “reality” as we physics types describe it. You may have heard the word “quarks”, but I’ll start this exploration a little higher up, at the enormous scale of the atom. (1)
Let’s talk atoms
Richard Feynman once told us that if he were going to preserve only one piece of scientific knowledge, it would be that the universe is made of atoms. These are not a modern conception; they were originally envisioned by the ancient Greeks to be the indivisible building blocks of the universe, from which everything else is constructed. You might want to think of them as Lego, but don’t get too attached to that image, because it’s not very useful in the end. At very least you need to think of them as more than just those single-bump legos. Those can be Hydrogen, though. For now.
Everything is made of atoms, but atoms are made of still smaller particles: protons, neutrons and electrons. In the technical lingo, atoms are not fundamental particles. They contain a veeeeeery tiny core made of protons and neutrons surrounded by a cloud of electrons. What defines the type of atom (or the Element) you are looking at is the number of protons in the nucleus. Scientists have “observed” 118 elements at last count, but you need a particle collider to make anything heavier than plutonium, which is the 94th.(3)
High school chemistry may have steered you awry, because along the way we still teach a model of the atom with the electrons orbiting the nucleus like planets in the solar system. It’s only a stop on the way to the final destination. The math is simpler and it’s easier to picture… which makes it a dangerously enticing model, because it is wrong. Unless you need to calculate a hydrogen spectrum, you should throw it out, because it’s going to make things harder from here on in.
The sneaky but vital electron
Here’s the sneaky quantum part: electrons don’t really exist, not the way that you and I do (or the chairs, or table, or computer). Now, they are REAL, but they sort of, erm… blip in and out of existence as they interact with things. When you aren’t looking, they aren’t exactly there. But when you do look, or feel, there they are again. Electrons are key to all of our experiences of the world, so don’t abandon them. Just don’t imagine them as little plinko chips bouncing around. That will take you down a completely wrong path for imagining the structure of the universe. (4)
Touch your fingertips together for a moment. Try warming your hands by rubbing them quickly. Now feel a couple of different textures around you. Each of those interactions is a situation where electrons on one set of atoms are interacting with electrons in your skin. The electron clouds around atoms can’t go through one another, so you don’t fall through the chair you are sitting on. They also let you touch things, and feel the edge of your body. What’s more, the messages that travel up your arms and deliver themselves to your brain do so via a cascade of electron interactions. The same can be said of the chemical processes that allow your body to move, breathe, and live. Texture, temperature, taste, sight – all our perceptions of the world are made possible by the fact that electrons are not permanently bound to their nuclei. They vibrate, they absorb and emit energy, and they get moving so fast that they pop right off one atom and move to another.
Nothing you see is standing still. All around us, electrons are careening around, being struck by photons, attaching to new atoms, raising and lowering their energy states and kicking out radiation as a result. No panicking, here! When I say “radiation”, I mean heat and light, not just the scary stuff that runs a nuclear reactor. The atoms and molecules containing the electrons are doing the same things. That’s why your guitar doesn’t stay in tune, why your glass of water evaporates, and why your broken-down-car’s brakes are worse after it has been sitting for several months. At the material level, everything is in constant motion.
Teeny-tiny quarks and charge
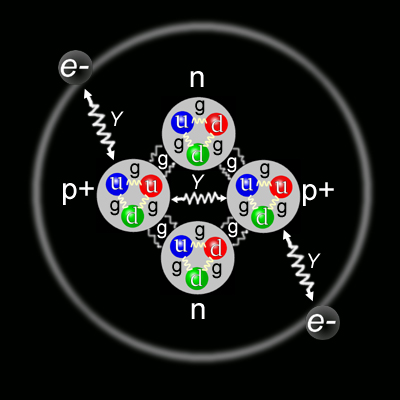
Which brings us back, indirectly, to gluons. If you recall, the electrons in atoms are flitting about in clouds around a nucleus that is made of protons and neutrons. As far as we know, electrons are fundamental – that is, they don’t break down into anything smaller, and they have no internal structure. Protons and neutrons, on the other hand, are built of still-smaller particles: the quarks.(5)
But wait! Before I can talk about gluons and what they have to do with quarks, I have to talk about charge.
Remember the electron? The electron has a charge that we define as “negative”. Negative charges do not like to be too close together – they repel one another. Protons also have “charge”, which we define as “positive”. (pro = positive) Stay with me here, because protons repel one another every bit as much as electrons do, but they are jammed together into a much, much smaller space. If an atom containing electrons is a football stadium, the nucleus containing protons (and neutrons) is a marble at the middle. (6) The atom is held together by the attraction between the electrons and the protons, because opposite charges attract.
Quarks also have charge. I suspect that if they had been imagined earlier, the whole definition of charge would be different, but they weren’t, and it isn’t, so quarks have fractional charges.(7) And when you combine three of them, they add up to either 1 (proton) or 0 (neutron). But here we have a problem – the nucleus is highly compacted, and highly charged. If the electronic forces were all that was at work, there could be no nuclei. The protons would blow apart and the universe would be a soup of structureless particles. The gluons hold it all together so that we get to eat, and breathe, and sleep, and make love. And dance!
Finally! With the gluons, and the dancing, and the hopping up and down!
Oh, I am so proud of you for staying with me so far! I tried not to put in too many numbers. Did it help?
In addition to the particles that make up matter, the Standard Model of Physics includes force-carriers. These are particles that are exchanged by bits of matter to… um… let them know where they are in space, how to stick together, and what types of particles they are, so to speak. Lets think of them as a conversation among the fundamental particles that tell them how to make themselves into protons and neutrons, how to combine to make atoms, how to be attracted together to make galaxies – in short, how to create the structure of the universe.
The gluons are only one of the charge carriers, but they have charge, and they have colour, and they have ephemeral existence, and are created by borrowing energy (very briefly) from the space-time continuum. They are SO COOL! They’re elegant. And they’re dynamic. And they, themselves are in a constant dance. In fact, what the standard model of physics tells me is that change and communication are the fundamental characteristics of the universe. We are in one giant sub-atomic cosmic dance with the stars, the galaxies, the protons, and the gluons, the cats, the chickens, the mountains, and the oceans. The dance is what is fundamental. So, you see, I just can’t help myself. It’s the way I am.
If you want to see an animation of this, have a look at this BestofScience video . The voice-over is a little irritating, and I’m not sure what’s up with the robotic scientist narrators, but the gluon part starting at 5:50 has pretty pictures.
1. The scales I used to work on were so small that I once complained (in all seriousness), “This model sucks. My atoms are moving around by an entire angstrom.” Reality check: That is 1/10th of a nanometer, or 1/10,000,000,000 th of a meter.
2. If you want a sweet song about the atoms and their classification, I can recommend They Might Be Giants song Meet The Elements. For older kids and adults, you might want to take a look at The Particle Adventure. I got my students to look at this as a pre-class activity. They had funding. It shows.
3. Also, most of them above Plutonium are only good for showing off how your particle collider is better than your colleague’s particle collider. I, myself, do not have a particle collider, so I may just have PC envy.
4. Don’t completely discount this approach, though. It’s a very good model for building electronics.
5. For our purposes, we are going to pretend that quarks only come in two “flavors”: up and down. There are four more, including the Strange one, but they are considered “exotic”. If you want more detail, check out the Particle Adventure.
6. I haven’t checked this calculation. I’m feeling lazy at the moment, and I’d like to get to gluons before I turn 40. And I still have to worry about Taekwondo, heteronormativity, and chickens in the chard, and that’s just today. Really, I’m just trying to explain why I was jumping up and down in class.
7. up quarks are +2/3 and down quarks are -1/3

4 responses to “Gluons Make me Dance”
Thanks for this! I may borrow parts of it when I’m teaching it to my class.
Very nice post. I am writing a book on evolution, but was devoting one chapter to atoms. I wanted to go a little into quarks and gluons and the standard model but found the information available very confusing. I think the aspect that I find most confusing is that many web resources parrot little quips about this particle or that, about quantum this or that. But I found very little adequately explained as far as the very scientific basics. Which particles, bosons and theories have been observed and are proven, and which aspects are models and theoretical in nature? Most of the concepts appear cloaked in higher math to the point of making particle physics truly appear similar to an arcane religion. I am about half way through with “Not Even Wrong” and “The Trouble with Physics” and these two sources seem to support a layperson’s skepticism. Is there a good source which clearly denotes which concepts and particles and force carriers have been observed and which aspects of the standard theories (and symmetry and strings, etc) are still just theories? For example the quote below. This is a lot for a layperson to digest. But which aspects have been clearly observed…how could you “Prove” these concepts to a layperson?
“but they have charge, and they have colour, and they have ephemeral existence, and are created by borrowing energy (very briefly) from the space-time continuum.”
Hmmm. I will have to think about that. Certainly the Particle Adventure (linked in the footnotes) is a good start. If you still want to know in a week, let me know. I’ll write a post called “How good is the standard model, anyway?” and find out some of those answers for you. I expect it will take a couple of weeks, though. FWIW, I haven’t even made it through “The Trouble With Physics,” even though it’s been on my end table for about two years.
Can I quote your comment in that post? It would be a great start to have the explicit questions.
[…] “quark” came. This is cross-posted from Seonaid’s other blog — “The Practical Dilletante.” […]